Calcium carbonate
Calcium carbonates may be considered
as materials of two families. One is natural calcium
carbonate, a ground limestone, used as
diluents to reduce compound cost. The particle
diameter is relatively large, ranging
from 1 to 5µm, and bulk density is low. Ground
calcium carbonates generally mix
easily into rubber. Good dispersion is obtained even at
very high loadings, up to 200 phr,
without significant increases in compound viscosity.
Although ground calcium carbonates
reduce cost, the magnitude of the practically
important volume cost reduction is
limited because of the high specific gravity.
The other family is precipitated calcium
carbonate. Limestone is burned in a kiln driving off carbon
dioxide gas and leaving calcium oxide.
The carbon dioxide gas is transferred to a calcium
oxide suspension to form calcium
carbonate again. The resulting carbonates are filtered,
dried, ground and separated by size. The particle size of
precipitated calcium carbonateis below 0.1 µm, thus the small particles are
reinforcing in rubber compounds.
Coating agents such as fatty acids or lignin
are used to prevent secondary flocculation.
Without coating, they tend to stick to the
walls of the internal mixer and become difficult to
disperse in rubber. Silane
modification of precipitated calcium carbonate can further
increase tensile strength of the
compound but not to the level achieved with a high surface
area carbon black.
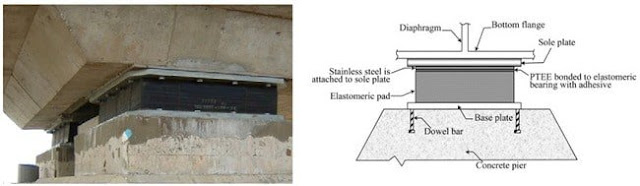
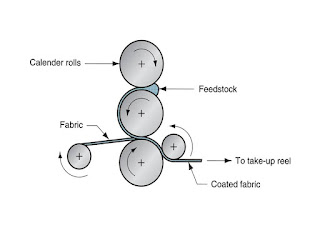

Comments
Post a Comment